Catalysis
Highlights in Catalysis > What is catalysis
This short description is adapted from that present in the web site of North American Catalysis Society and prepared by John Armor with suggestions from Bob Farrauto and Enrique Iglesia. It is intended to provide a short intro (for non-experts) to explain what catalysis is.
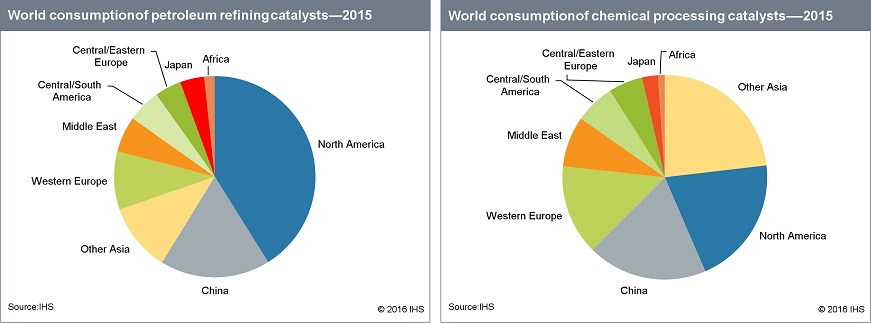
The economic contribution from catalysis is as remarkable as the phenomenon itself. Four sectors of the world’s economy are petroleum, energy production, chemicals production, and the food industry; together they account for more than 10 trillion dollars of the world’s GNP, and all of these are critically dependent on the use of catalysts. Estimates are that catalysis contributes to greater than 35% of global GDP; the biggest part of this contribution comes from the generation of high energy fuels (i.e., gasoline, diesel, hydrogen) which depend critically on the use of small amounts of catalysts in our world’s petroleum refineries. As a business, the catalyst market itself is growing from the current US$12 billion, so that catalysis costs are much less than 0.1% of the sales revenue from the products which they create.
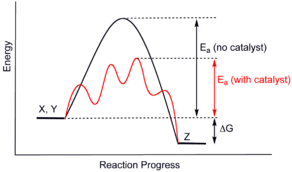
Catalysis is a technology which increases the rate of a chemical reaction. This technical field employs both scientists and engineers. Catalysts are the materials used by these persons to explore the phenomenon of catalysis. Catalysts are materials which speed up chemical reactions without the catalyst being consumed; they are materials which induce change. More specifically, catalysts are materials which change the rate of attainment of chemical equilibrium without themselves being changed or consumed in the process. Catalysts also provide selectivity or specificity to particular products which are more desirable than others. All these attributes about catalysis and catalysts translate to energy savings, less pollution, fewer side products, lower cost reactor materials, and ultimately products which reduce global warming. It has been said (A. Mittasch) that “chemistry without catalysis would be a sword without a handle…or a bell without sound.”

Catalysis is the key to both life and lifestyle. It is an essential technology for chemical and materials manufacturing, for fuel cells and other energy conversion systems, for combustion devices, and for pollution control systems which greatly impact everyone on our planet. Some other specific examples of what catalysts do include applications for:
- Fuels & Energy – Over half the world’s gasoline is currently produced by a process developed in 1942 called Fluid Catalytic Cracking (FCC). This process revolutionized the petroleum industry by more efficiently transforming higher boiling oils into lighter, usable products. FCC produces gasoline as well as heating oil, fuel oil, propane, butane, and chemical feedstocks that are instrumental in producing other products such as plastics, synthetic rubbers and fabrics, and cosmetics. It is considered one of the most important chemical engineering achievements of the 20th century. In the future, catalysts will be used to produce clean energy from renewable energy sources, such as hydrogen for fuel cells and transportation fuels from non-edible biomass.
- Emissions – Automobile emission catalysts have been developed since the 1960s to destroy CO, NOx and hydrocarbon emissions from mobile vehicles. Catalysts are also used to destroy the origins of sulfur based emissions in the combustion of fuels. In addition catalysts are widely used to destroy the objectionable emissions from the world’s coal fired power plants.
- Polymers – Catalysts are also used in the production of the world’s polymers. Current examples of polymers include adhesives, coatings, foams, and packaging materials, textile and industrial fibers, composites, electronic devices, biomedical devices, optical devices, and precursors for many newly developed high-tech ceramics.
- Life – Enzymes are one example of catalysts within our bodies which are critical to maintaining life. Further, the possibility of analyzing and ultimately manipulating genes rests on the catalytic properties of RNA to replicate molecules containing biological information.
- Health – The pharmaceutical industry employees large amounts of catalysts needed to produce the specificity of products they require. Catalysts used in the production of drugs are used to save lives and improve the health and lifestyle of people around the world.
- Food – Catalysts are widely used in food processing and enhance the performance of other consumer products such as laundry detergents.
The economic contribution from catalysis is as remarkable as the phenomenon itself. Four sectors of the world’s economy are petroleum, energy production, chemicals production, and the food industry; together they account for more than 10 trillion dollars of the world’s GNP, and all of these are critically dependent on the use of catalysts. Estimates are that catalysis contributes to greater than 35% of global GDP; the biggest part of this contribution comes from the generation of high energy fuels (i.e., gasoline, diesel, hydrogen) which depend critically on the use of small amounts of catalysts in our world’s petroleum refineries. As a business, the catalyst market itself is growing from the current US$12 billion, so that catalysis costs are much less than 0.1% of the sales revenue from the products which they create.

